Atoms and Elements
I. Atomic Structure
Matter is anything which takes up space and has mass. All matter on Earth is composed of elements. Elements are matter that can not be broken down into other substances by chemical reactions. An atom is the smallest unit of an element that retains all of the properties of that element. Glucose is a substance that is composed of three elements Carbon, Hydrogen, and Oxygen. There are 92 naturally occurring elements. Elements differ from one another in terms of the number of subatomic particles contained in these atoms. Atoms are generally composed of three different types of subatomic particles (Protons, Neutrons, and Electrons).
| Particle | Mass | Charge |
|---|---|---|
| proton | ~1 dalton | +1 |
| neutron | ~1 dalton | 0 |
| electron | ~0 dalton | -1 |
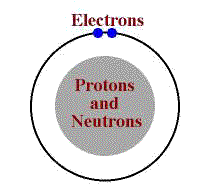
Protons are particles which have a mass of approximately 1 dalton (a unit of atomic mass) and a charge of +1.
Neutrons are particles which have a mass of approximately 1 dalton and no charge.
Electrons are particles with almost no mass (it is about 1/2000th of either a proton or neutron) and a charge of -1.
The protons and neutrons are found in the nucleus of the atom with the electrons orbiting this nucleus.
The atoms of different elements can be distinguished from one another on the basis of their Atomic Number (the number of protons in the nucleus) and Atomic Mass (the sum of the masses of all the subatomic particles in the atom).
Atomic Number = # of protons
Atomic Mass = is approximately the # of protons and neutrons
| Element | Protons | Neutrons | Electrons | Atomic Number |
Atomic Mass |
|---|---|---|---|---|---|
| Hydrogen | 1 | 0 | 1 | 1 | ~1 (1.008) |
| Helium | 2 | 2 | 2 | 2 | ~4 (4.0026) |
| Carbon | 6 | 6 | 6 | 6 | ~12 (12.0011) |
| Nitrogen | 7 | 7 | 7 | 7 | ~14 |
| Oxygen | 8 | 8 | 8 | 8 | ~16 (15.994) |
Try the following examples yourself.
II. Isotopes
Different forms of an element. Isotopes have the same atomic number, but a different atomic mass.
| Element | Protons | Neutrons | Electrons | Atomic Number |
Atomic Mass |
|---|---|---|---|---|---|
| Carbon | 6 | 6 | 6 | 6 | ~12 (12.0011) |
| Carbon 14 | 6 | 8 | 6 | 6 | ~14 |
Try these examples.
III. Valence and Valence Electrons
Niels Bohr (1913) developed a model of atomic structure to explain the observation that certain elements were highly reactive (H,C, etc.) and others (He, Ar, etc. ) were not. In this model electrons were moving in shells around the nucleus. The electrons always try to be in the lowest energy shell (that is closest to the nucleus). As electrons are added they fill the shells from the lowest to the highest in terms of energy.
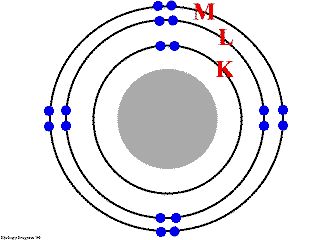 The lowest level
(K), can contain 2 electrons.
The lowest level
(K), can contain 2 electrons.
The next level (L) can contain 8 electrons.
The next level (M) can contain 8 electrons.
His next observation was that an atom was most stable (that is unreactive) when the outer most shell of electrons was full.
Atoms will try to gain or lose electrons in order to fill their outer shell or valence shell, the number of electrons to be gained or lost is called the valence. The electrons in the outer most shell are called the valence electrons.
| Element | Number of Electrons |
Number of Valence Electrons |
Valence |
|---|---|---|---|
| Hydrogen | 1 | 1 | 1 |
| Helium | 2 | 2 | 0 |
| Carbon | 6 | 4 | 4 |
| Nitrogen | 7 | 5 | 3 |
| Oxygen | 8 | 6 | 2 |
| Neon | 10 | 8 | 0 |
Try this examples.
Return to Table of Contents