Chemical Bonds
Chemical reactions result when two or more atoms come togther in such a way as to fill their outer electron shells (valence shells). Chemical bonds can be divided into those which are strong (Ionic, Covalent and polar Covalent) and those which are weak (hydrogen, hydrophobic interactions, Van derWaals forces).
I. Strong Bonds
The strong bonds are the types of bonds found within a molecule. These are the bonds that hold the atoms within a molecule together. Strong bonds are the result of electrical attractions between atoms.
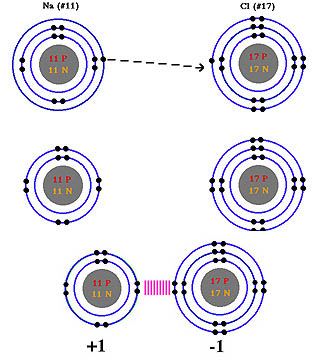
A. Ionic Bonds
In an ionic bond one atom has such a strong attraction for the electrons
(electronegativity) that it pulls an electron away from another atom.
This results in the two atoms now having charge (see the example of the
ionic bond found in NaCl).
The atom which gained the electron now has a charge of -1 (anion). The
atom which lost the electron has a charge of +1 (cation). These oppositely
charged atoms (now called ions) attract one another and as such tend to
stay together. This attraction is the ionic bond. Generally chemists consider
this to be the strongest of all bonds, as long as no water is present.
In biological systems, ionic bonds are weak due to this fact.
B. Covalent Bonds
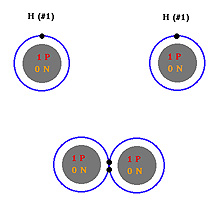 A covalent bond generally forms when two atoms of the same
element are bonded together (See hydrogen-hydrogen bond). Since the atoms
have the same attraction for electrons (electronegativity) neither atom
can completely remove an electron from the other and as a result they
share the electrons equally.
A covalent bond generally forms when two atoms of the same
element are bonded together (See hydrogen-hydrogen bond). Since the atoms
have the same attraction for electrons (electronegativity) neither atom
can completely remove an electron from the other and as a result they
share the electrons equally.
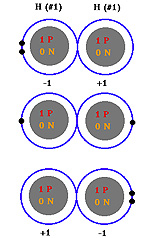 The bond forms as a result of the fact that the shared electrons
now orbit both atomic nuclei. Some of the time both shared electrons are
orbiting one nucleus, resulting in one nucleus having a momentary charge
of -1 and the other having a charge of +1. Again the oppositely charged
particles attract one another. Since the electrons are moving vey fast
they don't stay around this atom for long. In the next instant they may
both be found orbiting around the other atomic nucleus. This creates the
same attractive force except the atoms now have the opposite charges.
In the next instant the electrons may be found orbiting one atomic nucleus
each resulting in neither atom having a charge and momentarily no attractive
force. Covalent bonds are found between atoms of the same element. In
organic molecules there is one important exception to this. The bond between
hydrogen and carbon is also considered a covalent bond. This is because
hydrogen and carbon have similar electronegativity
.
The bond forms as a result of the fact that the shared electrons
now orbit both atomic nuclei. Some of the time both shared electrons are
orbiting one nucleus, resulting in one nucleus having a momentary charge
of -1 and the other having a charge of +1. Again the oppositely charged
particles attract one another. Since the electrons are moving vey fast
they don't stay around this atom for long. In the next instant they may
both be found orbiting around the other atomic nucleus. This creates the
same attractive force except the atoms now have the opposite charges.
In the next instant the electrons may be found orbiting one atomic nucleus
each resulting in neither atom having a charge and momentarily no attractive
force. Covalent bonds are found between atoms of the same element. In
organic molecules there is one important exception to this. The bond between
hydrogen and carbon is also considered a covalent bond. This is because
hydrogen and carbon have similar electronegativity
.
 Polar
covalent bonds are intermediate to ionic and covalent bonds (See H-O example).
Polar covalent bonds form between atoms of different elements and occur
when neither element has a strong enough attraction (electronegativity
) for the electrons to completley remove them from one another, but also
in which their attractions for the electrons are not equal.
Polar
covalent bonds are intermediate to ionic and covalent bonds (See H-O example).
Polar covalent bonds form between atoms of different elements and occur
when neither element has a strong enough attraction (electronegativity
) for the electrons to completley remove them from one another, but also
in which their attractions for the electrons are not equal.
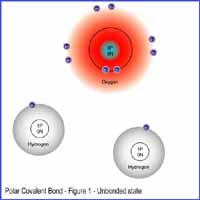
In Figure 1, we have an oxygen atom with 8 protons, 8 neutrons and 8 eletrons (2 in the first shell and 6 in the second shell.) We also have two hydrogen atoms floating around with 1 proton and 1 electron each. Click on Figure 1 to see enlarge image.
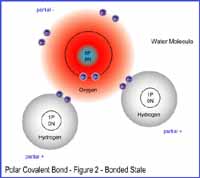 In Figure
2, the two hydrogens atoms bond with the oxygen atom to form a water molecule.
We can see the covalent bond between them because the hydrogens are sharing
their electrons with the oxygen atom.
In Figure
2, the two hydrogens atoms bond with the oxygen atom to form a water molecule.
We can see the covalent bond between them because the hydrogens are sharing
their electrons with the oxygen atom.
Click on Figure 2 to see its enlarge version.
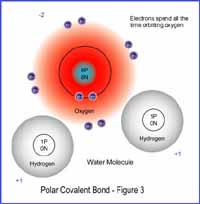 The result of this is that the electrons
spend more time orbiting one atomic nucleus than the other. One atom now
has a negative charge more than 50% of the time, but less than 100% and
the other atom involved has a positive charge more than 50% of the time,
but less than 100%. The bond is not ionic and not covalent, but rather
polar covalent.
The result of this is that the electrons
spend more time orbiting one atomic nucleus than the other. One atom now
has a negative charge more than 50% of the time, but less than 100% and
the other atom involved has a positive charge more than 50% of the time,
but less than 100%. The bond is not ionic and not covalent, but rather
polar covalent.
Click on Figure 3 to see high resolution view
How can you determine what type of bond will form between two atoms?
Here are a couple of simple rules. These rules are not accurate all the
time, but generally hold true.
1. Covalent bonds forms between atoms of the same element (C-C, H-H, O=O).
covalent bonds also forms between Carbon and Hydrogen (C-H).
2. Ionic bonds form between atoms of elements that come from the groups
at the extreme edges of the periodic table. , such as group I and group
VII elements (NaCl, ), remember the group VIII elements have full valence
shells and are generally unreactive.
3. Polar covalent bonds form between elements found in the groups in the
center of the periodic table, such as groups 3-6.
Try some examples.
II. Weak Bonds
Weak bonds are bonds that form between different molecules or within different parts of a large molecule. While these bonds are not strong enough to hold a molecule together they are extremely important because of their large number. There are three basic types of weak bonds (Hydrogen bonds, Hydrophobic Interactions and Van der Waals Forces).
A. Hydrogen Bonds
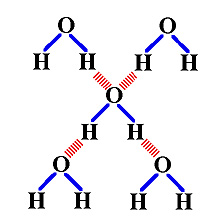 These bonds are the result of a hydrogen atom being bonded
to another atom (one with high electronegativity) via a polar covalent
bond. The resulting molecules have partial charges on different parts
of the molecule. In which the other atom has a strong attraction for electrons.
Although these bonds are very weak, in biological systems they are very
important.
These bonds are the result of a hydrogen atom being bonded
to another atom (one with high electronegativity) via a polar covalent
bond. The resulting molecules have partial charges on different parts
of the molecule. In which the other atom has a strong attraction for electrons.
Although these bonds are very weak, in biological systems they are very
important.
Water are a good example of
a molcule that readily forms hydrogen bonds. Each molecule contains polar
covalent bonds between the hydrogen atoms and the oxygen atom. The result
is that the oxygen end of each molecule has a partial negative charge and
the hydrogen end of each molecule a partial positive charge. These partial
charges attract one another resulting in a weak bond that holds molecules
of water to each other.
B. Hydrophobic Interactions
When non-polar substances such as fats or oils are placed in water they tend to clump together. This clumping of the non-polar substances is the result of hydrophobic interactions. Water is a polar compound as described above. As such water molecules interact with other polar molecules, but not with non-polar molecules. Compounds that mix well with water (polar) are known as hydrophilic (water-loving) those that do not mix well with water (non-polar) are known as hydrophobic (water-fearing). Molecules containing substantial non-polar regions will attract one another as a result of these hydrophobic interactions.
C. Van der Waals Forces
When molecules are in close proximity to one another very small attractions
can occur resulting from the constant movement of the electrons around
the atoms of the molecules. These very weak attractions are known as Van
der Waals Forces.
Try these examples.
Return to Table of Contents