Molecules, Compounds, and Chemical Reactions
I. Molecules and Compounds
A compound is a substance consisting of two or more different atoms, in specific proportions, bonded together in a specific pattern.
A molecule is the smallest part of a compound that retains all of the properties of that compound.
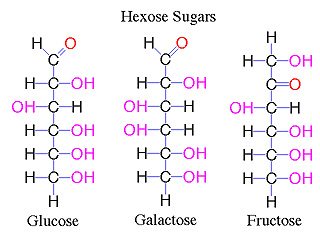
Molecular formulas are a shorthand way to represent the types and numbers of different atoms present in a molecule. Examples are H2O, water; CO2, carbon dioxide; C6H12O6, glucose, fructose, galactose.
Molecular formulas provide information on the proportion of atoms in a molecule, but not the pattern of bonding. While this does not cause a problem with small molecules (H2O or CO2) it can with larger molecules such as C6H12O6. This molecular formula is the same for the sugars glucose, galactose and fructose. Compounds with the same molecular formula, but different structures are called isomers. The figure below shows the isomers of the hexose sugars.
II. Molecular Weight and Avagadro's Number
The molecular weight of a molecule is equal to the sum of the atomic masses of all the atoms in a molecule
water H2O (1x2) + 16 =18 daltons
carbon dioxide CO2 (16x2) + 12 = 44 daltons
sucrose C12H22O11 (12x12) + (1x22) + (16x11) = 342 daltons
insulin C254H377N65O75S6 = 5727 daltons
Numbers of Molecules and Concentration
In most biological systems what we are usually concerned with is the number of molecules of a substance present, not the weight. To measure the concentration biologists use the concept of Gram Molecular Weight (Mole). The amount of a compound equal to its molecular weight in grams always contains the same number of molecules (6.023 x 1023, Avogadro's Number) 1 mole (symbolized by M).
18 grams of water contains the same number of molecules as
44 grams carbon dioxide
342 grams sucrose
5727 grams insulin
All of these quantities contain 1 mole of molecules
In solution the concentration of a compound is measured by the number moles/liter of solution (molarity). To prepare a 1 molar sucrose solution you would combine 342 grams of sucrose with enough water to make a final volume of 1 liter. It is important to remember that you do not add 1 liter of water to the sucrose, but rather enough water to make a final volume of 1 liter.
Try these examples.
II. Chemical Reaction
During chemical reactions one or more molecues may be converted into different molecule/s, but the total quantity of matter must remain the same. In the chemical reaction below note that there are the same numbers of each atom on both sides of the equation. This is true for all chemcial reactions.
Also note that in this reaction there are arrows pointing in both directions. Like most chemical reactions this one is reversible.
Return to Table of Contents